|
|
|
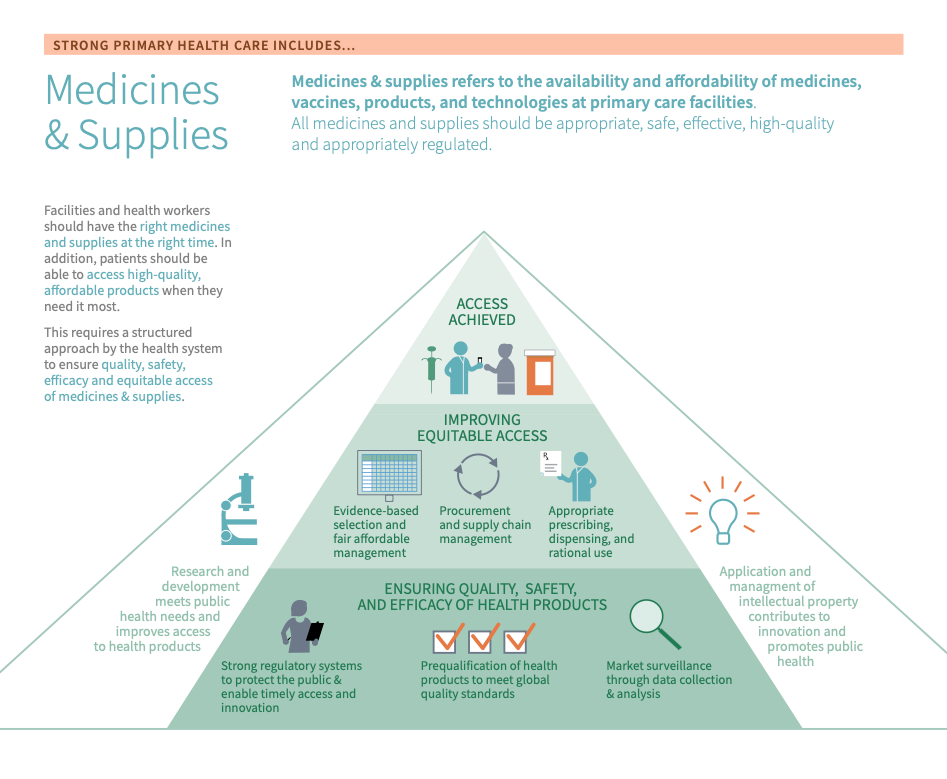
Medicines and Supplies refers to all medicines, vaccines, products, and technologies at primary care facilities. Medicines and Supplies assesses the availability and affordability of appropriate, safe, effective, and high-quality medicines and health products, including the appropriate regulation of such.
Strong primary health care is dependent on affordable, timely, and dependable access to safe, effective, and high-quality medicines and supplies.
Making sure facilities have the right medicines and supplies at the right time and that patients can access high-quality, affordable products when needed is imperative to delivering high-quality PHC services PHC services refer to any intervention, procedure, regimen, or process that providers use to respond to the needs and demands of their patient population at the primary care level. Because of PHC’s community-facing orientation, services can be provided virtually or face-to-face in homes, communities, or PHC centres. Depending on the context, services may be provided by public or private providers. . This requires a structured approach by the health system to ensure quality, safety, efficacy and equitable access of medicines and supplies.
Medicines and supplies are essential elements of all functioning health systems.12 Making sure facilities have the right drugs and supplies at the right time and that patients can access affordable products when needed is imperative to delivering high-quality primary health care and achieving the Sustainable Development Goals.
To achieve this, the World Health Organization (WHO) identifies two related core principles of focus: 1) ensuring quality, safety, and efficacy of health products and 2) improving equitable access. Each focal area includes a subset of strategic levers that can be employed to support strong Regulatory systems National regulatory authorities (NRAs) provide regulatory oversight of all medical products. They perform their mandate based on a legal framework and a set of recommended regulatory functions that span the medical product lifecycle including clinical trial oversight, marketing authorization and registration, licensing and inspection of premises, market surveillance and enforcement activities when required. , quality manufacturing, efficient supply chains, and effective post-market surveillance.1
Strong PHC that is capable of providing a comprehensive suite of promotive, preventive, and curative services is dependent on affordable, timely, and dependable access to safe, effective, and high-quality medicines and supplies. Countries often face a range of obstacles in achieving this, including rising prices for new medicines and supplies; shortages and stock outs of Essential medicines Essential medicines should satisfy the priority health care needs of the population. They are selected with due regard to public health relevance, evidence on efficacy and safety, and comparative cost-effectiveness, and compiled in the WHO Essential Medicines List. Essential medicines are intended to be available within the context of functioning health systems at all times in adequate amounts, in the appropriate dosage forms, with assured quality and adequate information, and at a price the individual and the community can afford. and health products - especially for noncommunicable diseases; and the growing problem of substandard and falsified medical products entering the global supply chain.2 Ensuring that the right high-quality medicines and supplies are available when needed in primary health care settings requires sufficient financing, effective management of funds at the facility level, appropriate physical infrastructure to store medicines and supplies, and information systems to track procurement and distribution. Additionally, appropriate use depends on provider competence and training.
PHCPI is a partnership dedicated to transforming the global state of primary health care, beginning with better measurement. While the content in this report represents the position of the partnership as a whole, it does not necessarily reflect the official policy or position of any individual partner organization.
Read on to learn how to use country data to:
- Make informed decisions about where to spend time and resources
- Track progress and communicate these updates to constituents or funders
- Gain new insights into long-standing trends or surprising gaps
Countries can measure their performance using the Vital Signs Profile (VSP). The VSP is a first-of-its-kind tool that helps stakeholders quickly diagnose the main strengths and weaknesses of primary health care in their country in a rigorous, standardized way. The second-generation Vital Signs Profile measures the essential elements of PHC across three main pillars: Capacity, Performance, and Impact. Medicines & supplies is measured in the Inputs domain of the VSP (Capacity Pillar).
If a country does not have a VSP, it can begin to focus improvement efforts using the subsections below, which address:
- Key indications
-
If your country does not have a VSP, the indications below may help you to start to identify whether medicines & supplies are a relevant area for improvement:
- A fragmented supply chain: if the supply chain for medicines and supplies is unreliable, poorly managed, or does not reach its end user then this could be an indication that improvements in medicines and supplies are needed
- An ineffective National Regulatory Medicines Authorities (NRMA): if NRMA is understaffed, underfunded, or otherwise unable to carry out its duties, then this could indicate a need for improvement
- Equipment and supplies do not meet quality standards: if diagnostic equipment, medicines, vaccines, immunization-related equipment, or medical devices do not meet the international standard then focusing on medicines and supplies will likely be beneficial
- No mechanisms in place for assessment: if there is no system in place to assess the adequacy of the supply chain, the regulatory committees, or the equipment and drugs, this indicates a need for improvement of medicines and supplies
- Resource shortages: if medicines and supplies are not evenly and equitably distributed throughout the population it may lead to resource gaps or shortages.
- Key outcomes and impact
-
Countries that strengthen the availability of medicines & supplies may achieve the following benefits or outcomes:
- Delivering high-quality PHC: ensuring facilities have the right drugs and supplies at the right time as well as ensuring patients can access affordable and high-quality products when needed
- Addressing inequities in access: working on improving medicines and supplies can help to ensure equitable access to affordable products and the equitable distribution of various products and supplies
- Supply management: working to ensure patients are receiving the right kinds of prescriptions and appropriate amounts of certain medicines
- Safety and effectiveness: employing safety mechanisms are helpful for the safe administration and use of medicines and supplies in the facility setting thus improving the overall safety of care. Additionally, adherence to established, evidence-based standards at the facility level is complementary to the effective and safe use of drugs and supplies for their intended purposes, again, promoting the overall safety of care
PHCPI is a partnership dedicated to transforming the global state of primary health care, beginning with better measurement. While the content in this report represents the position of the partnership as a whole, it does not necessarily reflect the official policy or position of any individual partner organization.
Explore this page for a curated list of actions to improve medicines & supplies, which embark on:
- An explanation of why the action is important for medicines & supplies
- Descriptions of activities or interventions countries can implement to improve medicines & supplies
- Descriptions of the key drivers in the health system that should be improved to maximise the success or impact of actions
Relevant tools and resources
Key actions:
-
Ensuring the quality, safety, and efficacy of health products is a critical component of delivering high-quality primary health care. Employing safety mechanisms lends itself to the safe administration and use of medicines and supplies in the facility setting, thus improving the overall safety of care for patients and providers.
This action provides guidance on the steps countries can take to better ensure the quality, safety, and efficacy of health products. It contains the following sub-actions:
Sub-action 1. Strengthen regulatory systems
Sub-action 2. Market surveillance and assessment of quality, safety & performance
Sub-action 1. Strengthen regulatory systems
National Medicines Regulatory Authorities are responsible for the safety, quality, and efficacy of medical products. An effective regulatory authority gives communities confidence that the products they need and use are safe and effective. Strengthening regulatory systems focuses on developing countries’ ability to deliver regulation that protects the public and enables timely access to, and innovation for, quality products.1
Key activities
- Promote innovation to foster R&D for affordable, suitable new treatments, diagnostics and devices1
- Utilize evidence-based selection for resource allocation to satisfy the priority health care needs of the population1
- Continuous support of the supply chain and governance1
- Strategic local and/or regional production of and health products1
- Promote affordability and fair pricing by pricing-financing reimbursement1
- Employ the quality use of medicines and health products, particularly antibiotics1
- The effective use of data systems to support decision-making and manage access to medicines, vaccines, and other health products17
Sub-action 2. Market surveillance and assessment of quality, safety & performance
A country’s capacity to collect, organize, analyze, and use quality data is a critical element of understanding the availability, quality, and safety of drugs and supplies on the market and taking corrective action when needed.
Key activities
- Use prequalification: “aims to ensure that diagnostics, medicines, vaccines and immunization-related equipment, diagnostics, and medical devices meet global standards of quality, safety and efficacy”1
- Provide a list of products that comply with unified international standards1
- Continuously monitor substandard or falsified health products1
- Collection of robust safety data and the development of pharmacovigilance infrastructure1
- Create a set of dedicated personnel to oversee supply chain implementation and monitoring1
- Implement technical guidelines: for quality assurance and to ensure the safety of medicines and supplies.18
- Periodic assessments/surveys: normalize periodic random surveys of storage, availability and quality of health products and supplies.18
Related elements
- Policy & leadership
- Information & technology
- Adjustment to population health needs
- Management of services
- Service availability & readiness
- Primary care Primary care is “a key process in the health system that supports first-contact, accessible, continuous, comprehensive, and coordinated patient-focused care.” functions
-
High-quality primary health care is dependent on patients and providers having access, regardless of geographic location, to safe, effective and quality medicines and supplies. It is critical that these health products are affordable, accessible and safe for every patient. Improving equitable access requires policy and programmatic efforts throughout the value chain from development through delivery.
Key activities
- Research & development that meets public health needs & improves access to health products: Health R&D is especially important for neglected diseases, emerging infectious disease pathogens, new antibiotic therapies, medical product innovations, and neglected target populations such as children and pregnant women. Effective research and development require setting priorities that align with population health needs and incentivizing investment in this area to ensure that available drugs and supplies keep pace with changing demand.1
- Application and management of the intellectual property to contribute to innovation and promote public health: Intellectual property (IP) protection has a large impact on innovation and access to health products. Activities related to this lever promote medical research and development, innovation, and increased access to drugs and supplies by incentivizing needs-driven innovation and access to affordable health products through appropriate trade and IP policies that reflect public health objectives.1
- Evidence-based selection and fair and affordable management:
Essential medicines
Essential medicines should satisfy the priority health care needs of the population. They are selected with due regard to public health relevance, evidence on efficacy and safety, and comparative cost-effectiveness, and compiled in the WHO Essential Medicines List. Essential medicines are intended to be available within the context of functioning health systems at all times in adequate amounts, in the appropriate dosage forms, with assured quality and adequate information, and at a price the individual and the community can afford.
and supplies are those that satisfy the priority health care needs of the population. They must be selected with regard to public health relevance, evidence of efficacy and safety, and comparative cost-effectiveness. When a drug or supply is considered a priority or essential, it is especially important to ensure fair pricing to alleviate the expensive burden of out-of-pocket payments. A fair price is one that is affordable for health systems and patients and that also provides a sufficient market incentive for industry to invest in innovation and the production of medicines. Countries can use the following lists to guide their procurement of drugs and supplies:
- WHO Model list of essential medicines: 21st list 2019
- WHO Model list of essential medicines for children: 7th list 2019
- Essential Medicines and Health Products: Prequalified Lists of Medicines
- Prequalified Vector Control Products List of Prequalified In Vitro Diagnostic Products
- Second Model List of Essential In Vitro Diagnostics
- Medical devices by health care facility level: health post and health centre
- Procurement and : The continuous supply of quality, safe, effective, and affordable drugs and supplies is one of the key building blocks of every well-functioning health system. Good procurement practices play a key role in securing affordable prices and ensuring adequate and timely supply, while good supply chain management ensures that quality products are available at all levels of the health system. This lever focuses on the need for improved capacity for procurement and supply chain management and for better data and market analysis to inform policy decisions. For more information on supply chain management, see the section “Take a Deep Dive”.1
- Establish a national capacity for availability: be able to respond to emergency needs for certain medicines, supplies or medical devices.18
- Supply chain workforce: ensure the staff at each facility is motivated and competent and can effectively carry out duties related to supply chain management.5
- Utilize a Logistics Management Information Systems (LMIS): to help facilitate supply and demand analysis and data tracking to help inform supply chain-related decisions.5
- Appropriate prescribing, dispensing, and rational use: Appropriate prescribing, dispensing, and use of medicines is essential for ensuring health impact and effective use of resources. Achieving this requires provider competence to reach accurate diagnoses, affordable and available medications, and adequate marketing, education, and promotion for appropriate use. More information on training and competence can be found in the provider competence Improvement Strategies module.1
Related elements
- Policy & leadership
- Information & technology
- PHC workforce
- Adjustment to population health needs
- Management of services
- Service availability & readiness
- Population health management
- Organisation of services
- Resilient facilities & services
Relevant tools & resources
- John Snow Inc, 2016: Getting the Products to the People: How Private Sector Solutions Can Strengthen Supply Chains for Public Health
- WHO, 2019: WHO essential medicines list
- WHO, 2019: WHO model list of essential medicines: 21st list 2019
- WHO, 2018: World Health Organization (WHO) core principles of focus for Medicines & Supplies
- USAID, 2022: Strengthening Pharmaceutical Systems
- WHO, 2015: Guidelines for Surveys on Quality of Medicines
- WHO, 2022: Essential medicines Essential medicines should satisfy the priority health care needs of the population. They are selected with due regard to public health relevance, evidence on efficacy and safety, and comparative cost-effectiveness, and compiled in the WHO Essential Medicines List. Essential medicines are intended to be available within the context of functioning health systems at all times in adequate amounts, in the appropriate dosage forms, with assured quality and adequate information, and at a price the individual and the community can afford. and health products - distribution
- Asian Development Bank, 2016: Better regulation of medicines means stronger regional health security
- The National Academies of Science, Engineering, and Medicine, 2018: Making medicines affordable
-
How effective are existing regulatory systems?
Effective are an essential component in ensuring the availability and quality of drugs and supplies. A particularly helpful tool in identifying gaps in the regulatory system is the WHO Global Benchmarking Tool (GBT), the primary means by which WHO objectively evaluates regulatory systems. It incorporates the concept of ‘maturity level’, allowing WHO and regulatory authorities to assess the overall maturity of a regulatory system on a scale of 1 to 4. The case study on Tanzania describes how the GBT can lead to system improvements.
In addition to completing the GBT, to start considering where regulatory system strengthening could occur, implementers can explore: What is our system of adherence to WHO guidelines and norms? Is there collaboration between regulatory systems and health systems? Is there a system in place to identify gaps in our supply chain management of drugs and supplies? How would our country fare in dispersing drugs and supplies in the event of a public health emergency?
Is there a country-wide supply chain model?
Having a clear understanding of the current state of the supply chain is the first step toward knowing what questions need to be asked to guide innovation and change. Effective supply chains not only help ensure commodity security but also contribute to the success or failure of any public health program that depends on drugs and supplies. Tools such as the Supply Chain Compass can provide a high-level diagnosis of supply chain maturity. Other assessment tools can help to further diagnose bottlenecks and pain points in the supply chain.
In addition to completing supply chain diagnostic tools such as the Supply Chain Compass, implementers may ask themselves the following questions to explore the state of supply chains in their country: Is there one central governing body or regulatory system that oversees the human and financial resources and operations of the supply chain? Is there a supply chain strategy? Is it being implemented? Are key performance indicators defined and used to monitor supply chain performance? How might the private sector bolster effectiveness in the supply chain?
What processes are in place for the evidence-based selection of drugs and supplies that are delivered in the PHC system?
Drugs and supplies, including medicines, vaccines, , and (the items and products identified as necessary to treat, cure, and/or prevent health priorities) are essential elements of all functioning health systems. As such, it is important to have a structured approach to ensure all essential drugs and supplies necessary for high-quality primary health care are available when needed.
To assess performance in this area, implementers might ask: are evidence-based essential medicines and health products lists in use? Is there a system for monitoring the need, availability and inventory of essential medicines and health products?
Is there a responsibility for market surveillance and assessment of quality, safety and performance of drugs and supplies?
As identified through the WHO's Roadmap for Access 2019-2023, a global priority is having regulatory systems prioritize authoritative guidance in meeting standards for ensuring drugs and supplies are accounted for, knowing if they are effective by the time they reach end-users, and subsequently taking action to improve the system. Surveillance The ongoing and systematic collection, analysis, and interpretation of health-related data essential to the planning, implementation, and evaluation of service delivery and public health. requires robust safety data and corresponding infrastructure for pharmacovigilance in support of the prevention, detection and response to substandard and falsified health products. As such, these systems require dedicated personnel to oversee supply chain implementation and monitoring.
Implementers may ask themselves the following questions to understand the state of market surveillance and quality assurance in their country: What data are being collected for surveillance and assessment of quality, safety and performance? Who is responsible for collecting this data and with which entities is it shared? Are there opportunities to improve efficiency in these processes?
Is there a systematic approach to supporting, supervising, and maintaining supply chain management (SCM) staff?
Because of the wide array of staff needed to ensure successful SCM, implementers at the management level of regulatory systems should be dedicated to estimating appropriate system-wide human resource needs, recruiting and deploying staff competent in supply chain and logistics tasks, and increasing the professionalization of these cadres to include data skills for continual market surveillance and assessment and evidence-based selection. Staff need to be supported by strong human resource policies and plans and supervised using performance management designed specifically to support them and build their capacity in SCM. The following resources have valuable insights on strengthening the human resources in the supply chain:
- People That Deliver
- Who is preparing the next generation of immunization supply chain professionals?
Implementers may ask themselves the following questions to further assess supply chain management in their country: What are the SCM tasks that need to be completed at various levels of the health system? What competencies are necessary for implementing these SCM tasks? Are the personnel assigned to these SCM tasks properly trained to complete them?
What is the role of the pharmacist in this country? Do pharmacists have the training, regulation, and incentives they need to perform this role?
In LMICs, the pharmacist’s role has changed significantly in recent years. The WHO identified appropriate prescribing, dispensing, and rational use as a lever for improving access and ensuring health impact and effective use of resources. Providing consumers with medicines alone is not sufficient to achieve treatment goals. Pharmacists now often hold responsibility for patient uptake and adherence in medication use; in order to fulfil this role, they must often be readily available to patients with or without an appointment and manage/triage health-related problems. To address these medication-related needs, pharmacists must accept greater responsibility for the outcomes of medicine use and are evolving their practices to provide patients with enhanced medicines-use services.3
Implementers can explore the state of the pharmacist role by asking: Are pharmacists integrated into care delivery networks to ensure coordinated and comprehensive patient care across settings and to promote primary health care as the first point of contact? Are pharmacists in this setting charged by the relevant authorities with the management of the distribution of medicines to consumers? Are they required to engage in appropriate efforts to assure the safe and efficacious use of medicines? Do they have the appropriate training to provide these functions? What regulations already exist to ensure the quality of pharmacist practice? What incentives do pharmacists have to perform this expanded role?
Is the need for assistive products being met?
Assistive products are any external product (including devices, equipment, instruments or software) with the primary purpose of maintaining or improving an individual’s functioning and independence, thereby promoting their well-being.4 These products range from a simple pair of eyeglasses (needed by 970 million people worldwide) to more complex technologies such as wheelchairs (needed by 75 million people).
Countries can use the WHO Priority Assistive Products List (APL) to determine which assistive products are needed. The list responds to a growing need for quality-assured, useful, and affordable assistive products as life expectancy increases across many regions and countries strive to fulfil global commitments to people living with disabilities.
PHCPI is a partnership dedicated to transforming the global state of primary health care, beginning with better measurement. While the content in this report represents the position of the partnership as a whole, it does not necessarily reflect the official policy or position of any individual partner organization.
Understanding and identifying the drivers of health systems performance--referred to here as “related elements”--is an integral part of improvement efforts. We define related elements as the factors in a health system that have the potential to impact, whether positive or negative, the distribution of medicines & supplies. Explore this section to learn about the different elements in a health system that should be improved or prioritized to maximize the success of actions described in the “take action” section.
While there are many complex factors in a health system that can impact medicines & supplies, some of the major drivers are listed below. To aid in the prioritization process, we group the ‘related elements’ into:
Upstream elements
We define “upstream elements” as the factors in a health system that have the potential to make the biggest impact, whether positive or negative, on the health workforce.
- Policy & leadership
-
The government plays a strong role in making medicines safe and high-quality via regulatory functions and investment in new technologies and research and development. Thus, weak or misdirected policies and leadership can threaten the quality, safety, and efficacy of essential medicines and supplies. For example, if governments do not follow global standards or routinely update their model list of essential medicines, population needs may go unmet. Furthermore, gaps in access to essential medicines may push patients to seek drugs elsewhere--putting them at risk of increased out-of-pocket spending and decreased pharmacovigilance. In addition, if regulatory agencies are weak or fragmented, it can compromise the quality, safety, and efficacy of the market. To be effective, regulatory agencies should ensure that only products of proven quality, safety, and efficacy are on the market along the entire supply chain. (Ball et al. 2016; National Academies of Sciences, Engineering, and Medicine et al. 2017) For example, mechanisms such as routine inspections and quality safety standards help to ensure integrated procurement and supply chain mechanisms for increased system efficiency and supply availability.
- Information & technology
-
Information systems play an important role in market surveillance, procurement, and supply chain management. Adequate information flow in supply chains is one of the most important issues for the supply chain management. When used effectively, information systems--especially logistics management information systems--ensure that supply chains are precise, rational, and respond to market demand. In addition, information systems that capture data on the safety, efficacy, and quality of medicines--such as adverse drug events--can help to improve medication safety. In addition, systems that capture and monitor data on the availability, pricing, and affordability of essential medicines can help policymakers and planners to better understand access issues from the patient perspective.12141516
- Organisation of services
-
The organisation of services helps to define which PHC services are supported and delivered by varying facility types, thereby influencing the necessary drugs and supplies needed at the point of service. Each health facility can come with a diverse set of needs, so ensuring the right medicines and supplies are accessible at a given facility is critical.
Complementary elements
We define “complementary elements” as the factors in a health system that have the potential to make an impact, whether positive or negative, on the health workforce. However, we consider these drivers as complementary to, but not essential to performance.
- Adjustment to population health needs
-
Adjustment to population health needs, particularly in the form of local planning, allows for the planning and procurement of medicines and supplies that are most needed for local facilities. For example, some countries have established systems to "pull" demand for medicines and supplies as they are needed by local facilities.
- Purchasing & payment systems
-
Purchasing & Payment systems often impact the acquisition of and investment into necessary medicines and supplies, however, it is not necessary that this alone would impact Medicines & Supplies. Similarly, changes to spending on PHC as a whole can impact the provision and availability of Medicines & Supplies, but again likely won’t impact this input alone.
Learn more in the Purchasing & Payment Systems or Funding & Allocation of Resources modules.
- Management of services
-
Given some facility funding is decentralized, facility management and leadership can be complementary to the procurement of drugs and supplies. Additionally, quality improvement at the facility level ensures quality standards for safety and efficacy in drug and supply procurement and use are followed.
- Service availability & readiness
-
Facility readiness for service delivery aids in the safe and appropriate administration and use of drugs and supplies in the facility setting. It means that providers are available, competent, and motivated to safely administer and use medicines and supplies at the facility level.
- Population health management
-
Understanding local priorities and local contexts is complementary in enabling the identification of necessary (and cost-effective) drugs and supplies.
- Resilient facilities & services
-
Assessments of resilience in service preparedness can help identify vulnerabilities in drug or supply chains that require improvement.
PHCPI is a partnership dedicated to transforming the global state of primary health care, beginning with better measurement. While the content in this report represents the position of the partnership as a whole, it does not necessarily reflect the official policy or position of any individual partner organization.
Countries seeking to improve their use of medicines & supplies can pursue a wide array of potential improvement pathways. The short case studies below highlight promising and innovative approaches that countries around the world have taken to improve.
PHCPI-authored cases were developed via an examination of the existing literature. Some also feature key learnings from in-country experts.
- East Asia & the Pacific
- Europe & Central Asia
- Latin America & the Caribbean
-
- Dominican Republic: Innovation in supply chain management (SCM)
- Middle East & North Africa
- North America
- South Asia
- Sub-Saharan Africa
PHCPI is a partnership dedicated to transforming the global state of primary health care, beginning with better measurement. While the content in this report represents the position of the partnership as a whole, it does not necessarily reflect the official policy or position of any individual partner organization.
Building consensus on what effective use of medicines & supplies looks like and key strategies to fix gaps is an important step in the improvement process.
Below, we define some of the characteristics of what effective use of medicines & supplies means in greater detail:
-
Equitable access is crucial as it ensures that all patients have access to affordable, high-quality products at any given moment, particularly when they need said supplies most. Improving equitable access requires policy and programmatic efforts throughout the value chain from development through delivery. This includes evidence-based selection, , and appropriate prescribing and use.1 Other levers and actions that are important in strengthening equitable access include: focusing on research and development that addresses the most pressing public health needs and effective utilization and management of intellectual property (IP) to contribute to innovative solutions and improve public health.
-
Access to medicines and supplies is critical, but these products must be of high quality and safe for use in the population. Ensuring the quality, safety, and efficacy of health products can be achieved through the following: strengthening like the NMRA, utilizing pre-qualification to assess the quality, safety, and performance of medicines, supplies and other health products, as well as using market surveillance to monitor quality, safety and performance.1 A country’s capacity to collect, organize, analyze, and use quality data is a critical element of understanding the availability, quality, and safety of drugs and supplies on the market and taking corrective action, as needed.
-
The supply chain refers to the resources needed to deliver goods or services to a consumer. In healthcare, supply chain management (SCM) involves obtaining resources, managing supplies, and delivering goods and services to providers and patients. Good supply chain management ensures that quality products are available at all levels of the health system. The continuous supply of quality, safe, effective, and affordable drugs and supplies is one of the key building blocks of every well-functioning health system. Good procurement practices play a key role in securing affordable prices and ensuring adequate and timely supply.
SCM activities include5:
- Serving customers – Customer needs are central to the selection, procurement, storage, distribution, and dispensing of products. Maintaining this focus and adhering to relevant protocols helps ensure that customers receive the right products at the right times.
- Product selection – Country committees, usually with membership drawn from regulatory agencies and regulatory professionals in pharmacy, medicine, and nursing practices, are typically the responsible group for decision-making regarding product selection. Often the outputs of this committee are national and essential supplies lists, typically developed following the essential medicine lists patterned on the World Health Organization (WHO) Model List.
- Quantification – Post-selection, product quantities and costs must be determined. Quantification ensures an uninterrupted supply of products by projecting the quantity and cost of the products needed by a health unit or program and determining when the products should be procured and delivered.
- Procurement – Once a quantification supply plan is complete, drugs and supplies must be procured. A strategic approach is critical to this process of procurement – through careful research, planning, monitoring and attention to applicable regulations, health systems and programs will enable timely and quality-assured supply procurement.
- Inventory strategy - Inventory policies are central to meeting supply chain objectives and enable organizations to balance supply and demand. Integrated inventory strategies define policies that guide product selection, quantification, and storage plans.
- Warehousing & distribution - After procurement, there must be a structured approach to the physical management of a product through each of the supply chains. This protects the item from environmental harm or handling to ensure its quality and condition upon use.
Underlying each of these supply chain elements are management components that support the operational functions5:
- Logistics Management Information Systems (LMIS) - A LMIS facilitates supply and demand data tracking and analysis, which informs supply chain decisions and future commodity-related logistics actions. LMIS are frequently used for facility operations such as ordering and replenishing supplies.
- Supply chain workforce - Ensuring an effective flow of public health supply chain logistics requires motivated and competent staff at all levels. Their performance must be supported and honed through supervision, continuous learning, and opportunities for professional development.
- Financing - Distribution and administration of finances affect all elements of the supply cycle. As such, drugs and supplies - and the supply chains that deliver them - need to be sufficiently resourced. Assuring a budget line item for health commodities and associated management is critical to the availability of drugs and supplies and smooth systems operations.
- Performance management - Continuous monitoring of the supply chain’s key performance indicators is important to rigorously evaluate the status, effectiveness, and efficiency of supply chain operations and gauge if adjustments are needed.
- Risk management – In an effort to hone a manager’s focus and efforts where they are most needed, risk management is a formal approach to recognizing and alleviating dysfunction within the supply chain.
The supply chain can also be an opportunity to engage the private sector. The following resources orient implementers to ways in which the private sector operates and how they can be engaged:
-
- Supply chain management Supply chain management encompasses the planning and management of all activities involved in sourcing and procurement…and all logistics management activities. Importantly, it also includes coordination and collaboration with channel partners, which can be suppliers, intermediaries, third party service providers, and customers. In essence, supply chain management integrates supply and demand management within and across companies. (SCM) - Supply chain management Supply chain management encompasses the planning and management of all activities involved in sourcing and procurement…and all logistics management activities. Importantly, it also includes coordination and collaboration with channel partners, which can be suppliers, intermediaries, third party service providers, and customers. In essence, supply chain management integrates supply and demand management within and across companies. encompasses the planning and management of all activities involved in sourcing and procurement…and all logistics management activities. Importantly, it also includes coordination and collaboration with channel partners, which can be suppliers, intermediaries, third-party service providers, and customers. In essence, supply chain management integrates supply and demand management within and across companies.5
- Regulatory systems National regulatory authorities (NRAs) provide regulatory oversight of all medical products. They perform their mandate based on a legal framework and a set of recommended regulatory functions that span the medical product lifecycle including clinical trial oversight, marketing authorization and registration, licensing and inspection of premises, market surveillance and enforcement activities when required. - National regulatory authorities (NRAs) provide regulatory oversight of all medical products. They perform their mandate based on a legal framework and a set of recommended regulatory functions that span the medical product lifecycle including clinical trial oversight, marketing authorization and registration, licensing and inspection of premises, market surveillance and enforcement activities when required.6
- Essential medicines Essential medicines should satisfy the priority health care needs of the population. They are selected with due regard to public health relevance, evidence on efficacy and safety, and comparative cost-effectiveness, and compiled in the WHO Essential Medicines List. Essential medicines are intended to be available within the context of functioning health systems at all times in adequate amounts, in the appropriate dosage forms, with assured quality and adequate information, and at a price the individual and the community can afford. - Essential medicines Essential medicines should satisfy the priority health care needs of the population. They are selected with due regard to public health relevance, evidence on efficacy and safety, and comparative cost-effectiveness, and compiled in the WHO Essential Medicines List. Essential medicines are intended to be available within the context of functioning health systems at all times in adequate amounts, in the appropriate dosage forms, with assured quality and adequate information, and at a price the individual and the community can afford. should satisfy the priority health care needs of the population. They are selected with due regard to public health relevance, evidence on efficacy and safety, and comparative cost-effectiveness, and compiled in the WHO Essential Medicines List. Essential medicines Essential medicines should satisfy the priority health care needs of the population. They are selected with due regard to public health relevance, evidence on efficacy and safety, and comparative cost-effectiveness, and compiled in the WHO Essential Medicines List. Essential medicines are intended to be available within the context of functioning health systems at all times in adequate amounts, in the appropriate dosage forms, with assured quality and adequate information, and at a price the individual and the community can afford. are intended to be available within the context of functioning health systems at all times in adequate amounts, in the appropriate dosage forms, with assured quality and adequate information, and at a price the individual and the community can afford.7
- Essential consumable commodities Essential consumable commodities are the items and products identified as necessary to treat, cure, and/or prevent health system priorities. These may include vaccines, contraceptives, malaria products, sterile gauze, disposable needles, etc. - Essential consumable commodities Essential consumable commodities are the items and products identified as necessary to treat, cure, and/or prevent health system priorities. These may include vaccines, contraceptives, malaria products, sterile gauze, disposable needles, etc. are the items and products identified as necessary to treat, cure, and/or prevent health system priorities. These may include vaccines, contraceptives, malaria products, sterile gauze, disposable needles, etc.8
- Basic equipment Basic equipment are the essential supplies needed for “the safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease" at all primary care facilities. - Basic equipment Basic equipment are the essential supplies needed for “the safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease" at all primary care facilities. is the essential supplies needed for “the safe and effective prevention, diagnosis, treatment and rehabilitation of illness and disease" at all primary care facilities.9
- Diagnostic supplies Diagnostic supplies are the materials needed to conduct essential diagnostic tests, with the aim of providing information on a patient’s condition for diagnosis, monitoring, screening, prediction, or prognosis purposes at the primary care facility level. - Diagnostic supplies Diagnostic supplies are the materials needed to conduct essential diagnostic tests, with the aim of providing information on a patient’s condition for diagnosis, monitoring, screening, prediction, or prognosis purposes at the primary care facility level. are the materials needed to conduct essential diagnostic tests, with the aim of providing information on a patient’s condition for diagnosis, monitoring, screening, prediction, or prognosis purposes at the primary care facility level.10
- Prequalification Prequalification of medicines and health products is a United Nation program managed by WHO. The purpose is to assess the quality, safety and efficacy of medicinal products and work with national regulatory authorities to support countries in building regulatory capacity through networking, training and information-sharing. - Prequalification Prequalification of medicines and health products is a United Nation program managed by WHO. The purpose is to assess the quality, safety and efficacy of medicinal products and work with national regulatory authorities to support countries in building regulatory capacity through networking, training and information-sharing. of medicines and health products is a United Nation program managed by WHO. The purpose is to assess the quality, safety and efficacy of medicinal products and work with national regulatory authorities to support countries in building regulatory capacity through networking, training and information-sharing.11
PHCPI is a partnership dedicated to transforming the global state of primary health care, beginning with better measurement. While the content in this report represents the position of the partnership as a whole, it does not necessarily reflect the official policy or position of any individual partner organization.
References:
- WHO. Draft road map for access to medicines, vaccines and other health products, 2019 – 2023. World Health Organization; 2018.
- WHO. WHO Medicines, Vaccines and Pharmaceuticals, Annual Report 2018. World Health Organization. 2018;.
- Introduction. Joint FIP/WHO guidelines on good pharmacy practice: standards for quality of pharmacy services. WHO. 2011;
- WHO. Priority Assistive Products List: Improving access to assistive technology for everyone, everywhere. World Health Organization. 2016;
- John Snow, Inc. The Supply Chain Manager’s Handbook: A Practical Guide to the Management of Commodities . John Snow, Inc; 2017.
- W H O. Regulatory system strengthening [Internet]. [cited 2019 Jul 17]. Available from: https://www.who.int/medicines/regulation/rss/en/
- W H O. Essential medicines [Internet]. [cited 2019 Mar 15]. Available from: https://www.who.int/topics/essential_medicines/en/
- USAID. The Logistics Handbook: A Practical Guide for the Supply Chain Management of Health Commodities. 2011;
- W H O. Medical Device [Internet]. [cited 2019 Jul 25]. Available from: https://www.who.int/medical_devices/full_deffinition/en/
- WHO. Fifty-first report of the WHO Expert Committee on Specifications for Pharmaceutical Preparations. Geneva; 2017.
- WHO. Prequalification of medicines [Internet]. 2013 [cited 2019 Sep 24]. Available from: https://www.who.int/news-room/fact-sheets/detail/prequalification-of-medicines-by-who
- Ball D, Parry J, Roth S. Better regulation of medicines means stronger regional health security. ADB; 2016 Apr.
- National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Health Care Services, Committee on Ensuring Patient Access to Affordable Drug Therapies. Making medicines affordable: A national imperative. Nass SJ, Madhavan G, Augustine NR, editors. Washington (DC): National Academies Press (US); 2017.
- Sun H, Chen X. Medical products supply chain and its information system design. YM. 2019;03(02):142–8.
- Babar Z-U-D, Ramzan S, El-Dahiyat F, Tachmazidis I, Adebisi A, Hasan SS. The Availability, Pricing, and Affordability of Essential Diabetes Medicines in 17 Low-, Middle-, and High-Income Countries. Front Pharmacol. 2019 Nov 19;10:1375.
- Yousefi N, Alibabaei A. Information flow in the pharmaceutical supply chain. Iran J Pharm Res. 2015;14(4):1299–303.
- World Health Organization. Towards Access 2030: WHO Essential Medicines and Health Products Strategic Framework 2016-2030. Lancet. 2017 Jan;
- WHO. Transforming Vision into Action: Operational Framework for Primary Health Care. WHO; 2020 Dec.
- Ndomondo-Sigonda M, Miot J, Naidoo S, Dodoo A, Kaale E. Medicines regulation in africa: current state and opportunities. Pharmaceut Med. 2017 Nov 3;31(6):383–97.
- WHO | Improving the quality of medical products for universal access [Internet]. [cited 2019 Jul 12]. Available from: https://www.who.int/medicines/regulation/fact-figures-qual-med/en/
- WHO. Primary health care: transforming vision into action - Operational Framework. Global Conference on Primary Health Care. 2018;
- WHO. WHO | Tanzania is first African country to reach an important milestone in the regulation of medicines [Internet]. 2018 [cited 2019 Jul 23]. Available from: https://www.who.int/medicines/news/2018/tanzani1st-african-country-to-reach-regulation-meds-milestone/en/
- Barillas E, Valdez C, Espinozai H. Integrated Pharmaceutical Supply Management as a Strategy for Strengthening the National Health System in the Dominican Republic. USAID SIAPS. 2012;
- Systems for Improved Access to Pharmaceuticals and Services (SIAPS) Program B. Promising Practices: Procurement. Management Sciences for Health. 2014;
